- Home
- Dr Sultan Linjawi
Type 1 Diabetes
Type 2 Diabetes
Prediabetes
Gestational Diabetes
- Diabetes Information
- Testimonials
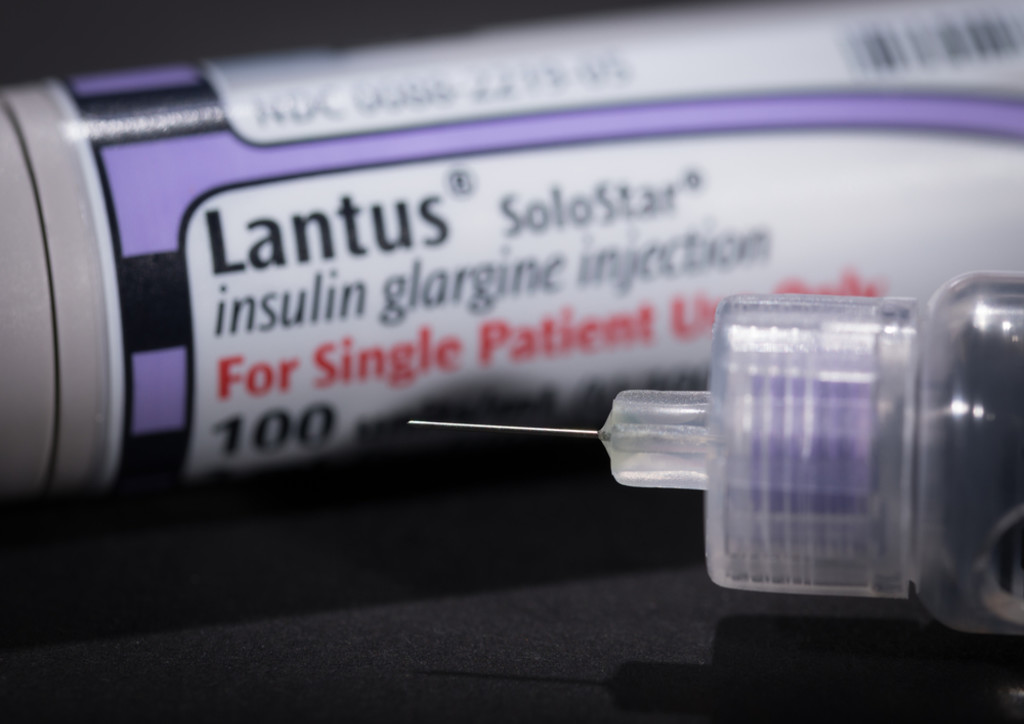
Lantus (long acting basal insulin glargine), what it does, how it works, is it for you and how to get the best results using it.
DO YOU WANT TO GET YOUR DIABETES UNDER THE BEST CONTROL?
Click the program that best describes your situation.
Lantus insulin is a long acting basal insulin analogue manufactured by Sanofi pharmaceuticals. It has been used widely around the world and was first approved by the FDA in 2000. It is approved for the treatment of type 1 and type 2 diabetes. The generic name for Lantus insulin is insulin glargine and recently a number of other manufactures have started to make and sell insulin glargine and sell it under their own brand name. Examples of other insulin glargine biosimilars are Optisulin, Basiglar, Abasaglar and Semglee.
Lantus insulin is promoted as being a once daily 24 hour insulin but there are lots of studies to suggest that for many people it is best administered twice per day, 12 hours apart (see below).
What is an insulin analogue?
Insulin is a small protein made up of multiple amino acids that are linked together. It is manufactured by beta cells in the islets of Langerhans in the pancreas. In humans insulin has a very short duration of action and is released continuously. When we eat foods that contain carbohydrates and sugars, a large spike of insulin is released.
By making small changes to the amino acids insulin manufacturers have been able to produce variations of human insulin called analogues that have specific characteristics that help with the delivery of insulin as a medicine. Modifications to insulins such as Humalog, Novolog/Novorapid, Apidra or Fiasp can for example when given before food speed up insulin delivery to help regulate blood sugars. It can also be more slowly released from under the skin so that insulin is absorbed continuously as a basal, long acting or background insulin.
What is a basal insulin?
Basal, long acting or background insulin is an insulin that when administered into the subcutaneous fat under the skin is absorbed slowly into the blood stream. There are many basal insulins listed in order of duration of action:
- Protaphane/NHP
- Levemir (insulin detemir)
- Lantus (insulin glargine U100)
- Toujeo (insulin glargine U300)
- Tresiba (insulin degludec)
Can Lantus be mixed with other insulins?
No. Lantus insulin in solution either in a vial or in a pre-filled solostar pen is very acidic at a pH of 4. This keeps it in a liquid state. When Lantus insulin is injected into the fat the pH changes to the body's neutral pH and Lantus insulin forms crystals. The acidic environment means that other insulins can not be mixed with it. You shouldn't mix it in a syringe or even inject a different insulin close by.
How long does Lantus work?
For most people Lantus works for over 24 hours but is only at the right level in the body for 16-20 hours. As the dose of insulin wears off the blood sugars may rise substantially for a few hours before the next dose. For this reason many doctors recommend splitting the dose and having them 12 hours apart.
Lantus, when injected, forms crystals under the skin. These crystals may vary in size and breakdown at different rates. This may lead to variable insulin release into the blood stream causing variable blood glucose levels. Using Toujeo insulin glargine improves this (see below).
What is the dose of Lantus?
The dose of insulin a person needs is different for every person. Typically the dose needed is the dose to achieve normal fasting blood glucose levels without the blood sugar going too low (hypoglycaemia).
The recommended starting dose of Lantus insulin (insulin glargine) is 10 units daily. There are many ways to increase the dose to lower glucose levels.
- increase 1 unit every day until fasting blood sugar 100 mg/dl or 5.5 mmol/l. or
- increase 2 units every 3rd day until fasting blood sugar 80-126 mg/dl or 4.4-7.0 mmol/l.
Should Lantus insulin be administered once or twice a day?
Whilst all of the Sanofi pharmaceutical sponsored clinical trials have focused on Lantus administration being only once a day and it is marketed heavily as a once per day insulin in reality you can take Lantus twice a day and many people need to. Some studies have suggested 30-40% of people require twice daily Lantus insulin (insulin glargine) in similar doses given 12 hours apart.
To reduce the risk of hypoglycaemia with Lantus the best time to take Lantus is at night before bed with the dose based on lowering the fasting sugar level the next morning. It is therefore important to record fasting blood sugar levels to ensure the correct dose of Lantus is being taken.
Why does Lantus insulin sting when it is injected?
Because Lantus insulin is dissolved in an acidic liquid with a pH of 4 in the pen, when it is injected under the skin it can sting. Injecting any acid would be similar but fortunately this discomfort only lasts a short while. Once injected the pH quickly neutralises.
Lantus may also sting if the amount of insulin injected is large in volume or if the insulin is injected into the skin itself rather than under the skin. This would result in a lump in the skin where insulin was injected. If using a 4mm needle sometimes changing to a 5mm needle may help reduce any stinging sensation.
Side effects of Lantus insulin Solostar pen
The main side effects of Lantus insulin are the same as for any insulin and these would include:
- low blood sugar or hypoglycaemia
- allergic reactions to the insulin or chemicals in the solution
- skin reactions
- development of lumps under the skin known as lipohypertrophy
- Weight gain
Toujeo vs Lantus - What is the difference between Toujeo and Lantus?
Lantus is insulin glargine in a concentration of 100U per ml. This means there are 100 units of insulin in every millilitre. Recently a more concentrated version of glargine has been released called Toujeo which has 300units of insulin in every millilitre.
A person on Toujeo insulin would inject the same number of units of insulin but the volume of liquid would only be 30% of the amount of Lantus injected.
As the insulin is more concentrated and a smaller volume is injected this does change some of the characteristics of the insulin and offer some advantages. The advantages of Toujeo U300 over Lantus U100 are as follows:
- longer effect
- more consistent release into the circulation
- 20-30% reduction in hypoglcaemia compared to Lantus
- Less stinging when injecting
- easier to inject and administer
How do you switch from Lantus insulin to Toujeo insulin
As Toujeo has a longer action the same amount of insulin is spread over a longer period of time. This results in a higher dose of insulin needed with Toujeo as compared to Lantus. In clinical studies people on Toujeo U300 required 10-20% more insulin compared to Lantus insulin.
Tresiba (insulin degludec) vs Lantus insulin
Tresiba is a new insulin developed by NovoNordisk. It is a very a long acting basal background insulin that is released into the blood stream in a very consistent way. Studies comparing Lantus Insulin to Tresiba have demonstrated that people using Tresiba (insulin degludec) have a 30-50% lower rate of hypoglcaemia (low blood sugar) compared to Lantus and this was most obvious through the night.
The dose of Tresiba insulin and Lantus insulin is the same so changing from Lantus is simple. The very long action of Tresiba means that it takes 5 days for a new dose to settle (compared to 3 days with Lantus).
Tresiba can be combined with rapid acting Novorapid (Novolog) in a 70%/30% ratio. This insulin is called Ryzodeg insulin (to learn more go to the Ryzodeg page).
Are Optisulin, Basiglar, Abasaglar and Semglee interchangeable with Lantus?
The simple answer is yes mostly they are. Optisulin is made by Sanofi who make Lantus insulin. It is exactly the same insulin and is a rebranding to compete with other generic insulins being sold at lower prices (Think of expensive clothing brands sold at factory outlets, same brand made in same factories but lower prices). The evidence for other generics, however, is based on studies with only a small number of people so it might be that we will discover in time that there are some differences with Basiglar, Abasaglar and Semglee. For this reason these insulins are called biosimiliar rather than bioidentical.

Is Lantus insulin (insulin glargine) safe to use in pregnancy?
The answer is probably although no studies have formally tested this and so Lantus insulin is not officially indicated for use in pregnancy. That being said many doctors do prescribe Lantus to pregnant woman needing insulin and so far there has been no issues reported to suggest any concern.
A reasonable approach is to continue to use Lantus insulin in woman with type 1 diabetes but to use approved insulins for women with gestational diabetes requiring insulin.
Levemir (Insuin detemir) is approved for use in pregnancy by the FDA and EMA and trials are ongoing looking at the safety for Tresiba (insulin degludec).
Does Lantus insulin need to be refrigerated?
Yes. If you have excess Lantus insulin injectable pens at home, they need to be stored in the fridge.
The best place to store your Lantus insulin pens in the fridge, is on the top shelf of the fridge door. The butter compartment is usually the safest place. You shouldn’t store your ozempic pens in the coldest part of your fridge. If the Lantus insulin injectable pen becomes too cold, then the Lantus will degrade, making it less effective, leading to higher blood glucose levels.
Long term storage should be in a fridge between 2-8℃ (36-47℉). Once the pen is used it can be safely used for 56 days if maintained at a comfortable room temperature of 15-30℃ (59-86℉).
Read the insulin storage article for more information.
How long can unopened Lantus be unrefrigerated?
Like many insulin products they are able to used effectively once out of the fridge. Generally the insulin is safe and effective for 30 days unrefrigerated as long as it is not exposed to temperatures that are too cold (less than 2C / 36F) or too hot (more than 30C / 84F).
If insulin is exposed to temperatures in the fridge that are too cold and it freezes the insulin will degrade quickly, won't work as well and the users blood sugars will climb unexpectedly. Correct storage of insulin is dicussed in the following article, Where do you store your insulin?
Looking for more diabetes videos?
Check out our latest diabetes videos for more great content.
More Articles on Diabetes Treatments
For more information about different Diabetes Treatments, please follow the links below.
Oral Medications for Diabetes
- Starting on Metformin. What you need to know.
- Benefits of Metformin… A Wonder Drug?
- Metformin in Pregnancy. Is it safe?
- Metformin and diarrhoea.
- Vitamin B12 deficiency and diabetes.
- What is an SGLT2 inhibitor? It's a novel way to lower blood sugars, lose weight and maybe live longer.
- Rybelsus (Semaglutide) - A new oral medication to treat Type 2 Diabetes.
---
Insulin for Diabetes
- What is Ryzodeg insulin and how do I use it?
- Fiasp insulin. Fast acting insulin aspart.
- Managing Diabetes with Insulin: Is it necessary?
- Lantas. Solostar insulin glargine.
---
Injections for Diabetes
- What is Trulicity (Dulaglutide) and how does it work?
- Ozempic (Semaglutide) is a new treatment for type 2 diabetes.
- Xultophy 100/3.6 - Type 2 diabetes medication - Benefits, Side Effects and How to Use It.
- What is glucagon and how can a glucagon kit help someone with diabetes?
---